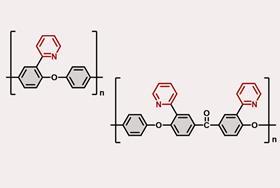
A thermoplastic, composed primarily of poly(aryl ether), has been developed that maintains strength during regular use but selectively breaks down when exposed to a nickel catalyst.
Poly(aryl ether) is well-suited to withstand heat and chemical exposure, making it ideal for demanding applications in packaging, automotives and electronics. However, its inherent durability makes it challenging to recycle mechanically. Poly(aryl ether)’s durability stems from strong C–C and C(aryl)–O bonds in its backbone, which make chemical recycling tricky too. To date, researchers have employed multi-step post-polymerisation modifications, including C–H oxidation and dehydrogenation to promote C–C bond cleavage, but such processes tend to yield a mixture of products unsuitable for repolymerisation. Alternatively, ‘we typically insert labile groups into polymer backbones so we can break them down into monomers, but this also causes issues of backbone stability,’ says polymer chemist Ying Yang from the University of Nevada, US, who was not involved in the research.
Researchers at Osaka University in Japan, led by Mamoru Tobisu, have overcome these issues by incorporating a directing group into the structure of poly(aryl ether). This directing group is a heteroatom-containing functional group that acts much like a lock: it maintains the polymer’s structural integrity under normal conditions but cleaves the backbone in the presence of a specific nickel catalyst by forming a stable metalacyclic intermediate. The polymer then breaks down cleanly and predictably, allowing it to be recycled without losing its inherent strength. ‘We’ve been developing catalytic methods to cleave strong bonds in small organic molecules for some time, and the use of directing groups is a well-established strategy in that area, says Tobisu. ‘Extending this approach to polymer recycling wasn’t a huge leap, but rather a Columbus’ egg moment – it was simple once we saw it.’
To test the concept, Tobisu’s team synthesised two types of poly(aryl ether)-based polymers using this directing group. They exposed the polymers to a nickel catalyst in the presence of a reducing agent and found that the polymers selectively degraded into their original monomers. The degraded monomers could then be reassembled into the original polymers, demonstrating potentially infinite recyclability without loss in material quality. The polymers also retained impressive thermal and chemical stability, with decomposition temperatures ranging from 399 to 465°C and high resistance to acidic, basic and oxidative conditions.
‘The study is a great starting point for making high-performance engineering polymers recyclable without altering the backbone structure or stability,’ says Yang. ‘It expands the toolbox of chemistries that are available … starting from here they can work on different backbone structures and extend the application to different types of polymers.’
The researchers will now be focussing on applying the design to other polymers, but to make the approach commercially viable, Tobisu says they’ll ‘need to substitute the current catalyst and reducing agent with more cost-effective alternatives’.
