Sepsis is a serious medical condition that occurs when the body has an extreme response to an infection. This reaction can lead to damage to the organs and, unfortunately, high rates of death, especially in critically ill patients in intensive care units. Diagnosing sepsis quickly and accurately is crucial, but current methods often struggle due to the complexity of the condition and the need for a lot of data. A new study at Jinan University introduces an innovative approach that could help improve sepsis diagnosis using advanced technology.
What Is This New Approach?
The researchers developed a deep learning framework called scCaT, which stands for “single-cell Capsulating Architecture with Transformers.” This model is designed to analyze genetic data from individual cells (single-cell RNA sequencing) and apply this information to larger groups of cells (bulk RNA data).
Capsulating Architecture: This part of the model groups genes together based on their biological functions. Think of it as sorting different tools in a toolbox by what they do—this makes it easier to understand how the genes are working together. This grouping not only helps the model learn better but also makes it easier for researchers to interpret the results.
Transformer Decoder: The Transformer component of the model helps classify whether a patient has sepsis or is healthy. It takes the organized genetic information from the capsulating architecture and uses it to make predictions.
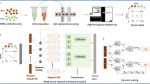
Study workflow diagram
A. Single-cell gene expression of peripheral blood mononuclear cells collected from sepsis patients and normal controls. B. Deep neural network architecture of scCaT. scCaT was constructed by blending capsule network and Transformer, and then it was trained using the gene expression of cells. C. Dynamic routing procedures of scCaT. D. Transfer learning. scCaT was transferred to subjects using bulk RNA data for fine-tune. It was evaluated and compared on independent cohorts.
How Effective Is This Model?
The scCaT model has shown remarkable accuracy in its predictions. Here are some highlights:
It achieved an AUROC score of 0.93 on tests using single-cell data. AUROC (Area Under the Receiver Operating Characteristic curve) is a measure of how well a model can distinguish between two groups—in this case, patients with sepsis and those without.
For bulk RNA data (which includes genetic information from larger samples), the model had an average AUROC score of 0.98 across seven different patient groups.
These high scores suggest that the model is very effective at identifying sepsis.
What Can This Model Do?
One of the key benefits of the scCaT model is its ability to recognize different types of cells and understand their biological pathways. This means it can not only identify if a patient has sepsis but also provide insights into how different genes and cells are involved in the disease process.
Moreover, this approach could be beneficial for diagnosing other rare diseases, especially where there is limited data available. By learning from the genetic information of individual cells and applying that knowledge to larger data sets, the researchers hope to improve the understanding and treatment of various conditions.
Conclusion
The development of the scCaT deep learning framework represents a significant step forward in sepsis diagnosis. By combining advanced machine learning techniques with biological data, this model enhances both the accuracy of sepsis detection and the ability to explain its findings. As these researchers continue to refine this technology, it could lead to better outcomes for patients suffering from sepsis and other challenging medical conditions. This innovative approach highlights the potential of using deep learning in healthcare to transform how we diagnose and treat diseases.
