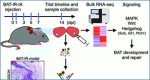
The prevalence of obesity worldwide has led to an increase in the risk of metabolic diseases and socio-economical burdens. Brown adipose tissue (BAT) has been established as a promising therapeutic target to overcome obesity, Type 2 diabetes (T2D) and other metabolic syndromes. Unfortunately, BAT degeneration occurs during aging and in response to diet-induced obesity. It has been unclear to what extent the adult BAT can regenerate and what are the cellular and molecular mechanisms underlying the regeneration.
To address these questions, researchers at Zhejiang University and Purdue University first established a novel and robust BAT degeneration model in adult mice. They discovered that BAT can efficiently regenerate itself within 14 days after phosphatidylcholine/ sodium deoxycholate (PC/NaDC) induced degeneration. The tissue can also regenerate after diphtheria toxin induced cell ablation. These results demonstrate that adult BAT is highly plastic.
Taking advantage of this model, they used single cell RNA-seq (scRNA-seq) to dissect the cellular dynamics during degeneration and regeneration of BAT. The scRNA-seq and functional analyses identify distinct progenitor populations contributing to BAT regeneration. These include the well-established PDGFRa+ fibroadipogenic (FAP) progenitors and novel populations of myeloid progenitors. These specific cell types express lipogenesis-associated genes after BAT injury, may act as progenitors of brown adipocytes and participate in BAT regeneration. The lineage tracing studies further confirmed contribution of FAPs and myeloid-derived cells to BAT regeneration. Therefore, multiple progenitor cell populations contribute to BAT regeneration.
To explore the molecular mechanisms underlying BAT regeneration, they performed bulk RNA-seq in addition to the scRNA-seq, this led to the identification of several signaling pathways that are enriched during BAT regeneration. From these pathways, the investigators focused on the Hedgehog (Hh) pathway, as previous studies have shown that Hh activation inhibits adipocyte differentiation and tissue regeneration. They found that the suppressor of fused (Sufu), an inhibitor of Hh in mammals, was activated after BAT injury. The scientists hypothesized that the upregulation of Sufu in progenitor cells would attenuate Hh signaling to facilitate BAT regeneration. To test this hypothesis, they used a gene ablation model to demonstrate that elevation of Hh signaling by reducing Sufu levels in brown adipocyte progenitors impairs BAT development.
The establishment of the BAT regeneration model and the identification of the cellular and molecular mechanisms underlying BAT regeneration open a new window to understand BAT plasticity and remodeling in the adults. The knowledge may lead to development of therapies to stimulate the expansion and thermogenic functions of BAT to combat obesity, diabetes, and other metabolic-related diseases in the future.
Source – Eurekalert
You W, Xu Z, Chen W, Yang X, Liu S, Wang L, Tu Y, Zhou Y, Valencak TG, Wang Y, Kuang S, Shan T. (2024) Cellular and Transcriptional Dynamics during Brown Adipose Tissue Regeneration under Acute Injury. Research (Wash DC) 6:0268. [article]
